
Regulatory affairs specialists will be at your site and guide you through the complex regulatory world. Our team will assist you with the planning, organizing and compiling of various regulatory based documents as well as with management of any necessary and arising post-approval queries and activities.
A various number of documents need to be compiled and submitted for the application and maintenance of marketing authorisations of healthcare products - which vary depending on the product category.
We support in the evaluation and preparation of the documents that have to comply with highest quality standards to be submitted and approved from various worldwide regulatory authorities. We also assist in preparing/compiling documents that have to be prepared in compliance with the standards and internal guidelines stipulated by the clients/sponsors.
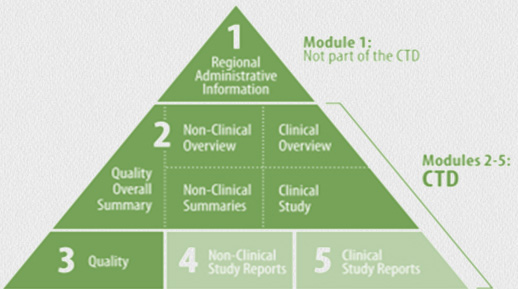
We can compile a complete dossier (module 1-5) or support in compiling individual modules. We revise, evaluate and maintain all sorts of dossier related documents. The dossiers comply with the high quality standards and requirements of the European authorities and the international standards of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
eCTD, NeeS, vNeeS and Paper- we can provide you dossiers compiled in any of the submission formats.
Evaluation of new or existing regulatory documents:
Preparation of the quality dossier
Structured Product Labelling (SPL) is a standard used by the FDA community to facilitate the communication of drug Labelling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. Since 2005, the FDA CDER division has provided guidance that all human prescription products be provided in SPL. Other FDA divisions have required SPL filing since June of 2009, including:
professional team is expert in offering comprehensive SPL conversion services to handle all product types. Our SPL conversion services include prescription drugs, OTC products, veterinary medicine, homeopathic products, and bulk ingredients. We also provide consulting and project management services for organizations that have or plan to develop internal capabilities. This hybrid approach will ensure your project is planned effectively and efficiently. We will be available to train and mentor your internal team throughout the project development.
The FDA now requires pharmaceutical manufacturers and distributors to report their distributions of drug samples under the Drug Sample Transparency Act (ACA 6004) specification. Like SPL, reporting is done using XML and through the FDA’s Electronic Submissions Gateway. DCL can review your ACA 6004 data on the distributions, and either converts it to XML as is or, as with SPL, provide a workbook for you to enter the data. You can then either submit the XML through the gateway or have us submit it for you.